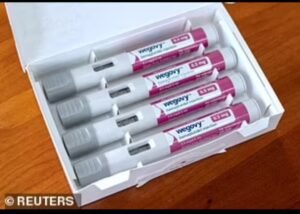
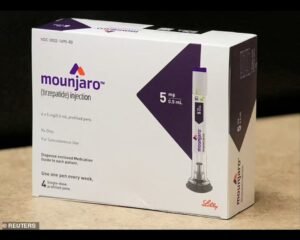
A wave of legal action is sweeping pharmaceutical heavyweights Novo Nordisk and Eli Lilly as dozens of patients pursue lawsuits over severe side effects linked to popular weight loss drugs, including Ozempic, Wegovy, and Mounjaro. The lawsuits allege that the companies omitted crucial warnings about the risk of gastroparesis, resulting in life-changing consequences for users.
Patients, including a woman facing a lifetime of diarrhea, are suing Novo Nordisk and Eli Lilly, claiming the weight loss drugs caused debilitating stomach paralysis.
Cameron Stephenson, an attorney at Levin Papantonio Rafferty, told DailyMail.com his firm currently has around 100 clients who were diagnosed with gastroparesis after using the drugs, and it is investigating 1,000 more.
Stephenson alleged that the companies sought initial approval for the drugs as diabetes treatments with the intention of promoting them for weight loss.
‘I expect to see documents to suggest that there was going to be off-label marketing and promotion outside of diabetes,’ he said.
‘I expect that the clinical trials and the things that they did in the various phases to come up with these drugs before they filed with the FDA, I think that they’re going to show that there was a risk of gastroparesis, and it’s not in the label, and it’s still isn’t in the label.’
Ozempic has FDA approval for the treatment of type 2 diabetes but has been prescribed off-label for weight loss to millions of Americans. Mounjaro also has approval for diabetes but can also be prescribed off label for weight loss.
Novo Nordisk’s Wegovy has FDA approval for chronic weight management.
Details:
Individual Cases:
- Brea Hand suffered from nausea and vomiting, leading to a gastroparesis diagnosis after using Ozempic. Hand, a mother-of-two who was prescribed the drug to control her fluctuating weight and pre-diabetes, required five hospital visits before physicians diagnosed her with gastroparesis and diabetic ketoacidosis, which can be life-threatening.
On her final hospital visit, she was admitted to intensive care.
‘They said my body was so acidotic that if I would have waited one more day that I wouldn’t have made it through,’ said Hand, whose lawsuit was filed on December 28.
- Robin Kelly, a teacher’s assistant, claims Ozempic caused violent illness and accuses Novo Nordisk of inadequate warnings. Robin Kelly, 49, filed a suit against Novo Nordisk in her home state of Mississippi on November 28, alleging that she became violently ill with gastroparesis after using Ozempic. She said she was not made aware that the condition was a possible side effect.
- Zakareeya Gregory faced gallbladder removal due to Ozempic side effects, experiencing severe stomach pain even after stopping the drug. Zakareeya Gregory, 46, from Walker Mill, Maryland, told DailyMail.com that her gallbladder was removed after she suffered side effects which were allegedly caused by Ozempic, which she used for seven months until February 2020. She has since regained the weight that she lost and said she also suffers from gallbladder attacks, crippling pains which can still occur after the organ has been removed.
- Billie Farley, 47, filed a suit against Novo Nordisk in December after using Ozempic for around three months.
Farley was hospitalized in January 2023 and a CT scan revealed ‘a life-threatening bowel injury’ which required extensive surgery that lasted nearly nine hours. Farley was told that the pain she continued to suffer ‘would be permanent for the rest of her life’.
‘She was told by the doctors that Ozempic had been the probable cause of her bowel injuries and to stop taking it immediately,’ the suit said. ‘She has not had a solid bowel movement since her surgery and has been advised by her medical professionals that she will never had a solid bowel movement again for the rest of her life.’
Legal Landscape:

Pharmaceutical Response:
Credit: Daily Mail